
PURPOSE: To demonstrate features of the burning process and to debunk myths about this supposedly well-known demonstration.
DESCRIPTION: A common pre-college experiment is to burn a candle inside of a bottle which has been turned upside down over a container of water. The water supposedly rises about one-fifth of the way up the bottle, indicating that the oxygen, about one-fifth of the air in the atmosphere, has been "used up" in the combustion process.
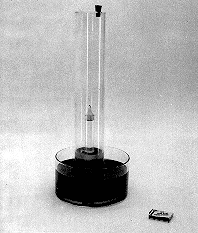
The candle in our experiment is placed inside the sealed tube containing air above a colored water bath, and is then ignited by a hot wire. The water level goes down initially, then returns to its original level just after the candle goes out. There is NO CHANGE in the water level from before to after the experiment is performed.
This is the CORRECT WAY to do this experiment. Two experimental errors are often made when performing this experiment: (1) if the candle is lit before the bottle is placed over it, the air is initially hot, and will pull the water up the bottle as it cools, and (2) when the bottle is placed over the candle, the hot air from the candle flame expands, and some of it might escape out of the bottom opening of the bottle. Analysis of the chemistry of this experiment shows that the final products of combustion are actually more voluminous than the initial air, but other things happen to yield no net difference in the water level.
SUGGESTIONS:
REFERENCES: (PIRA unavailable.) See Demonstration Reference File for analysis of the chemical processes during this experiment and lots of papers describing this experiment correctly and incorrectly performed.
EQUIPMENT: Burning candle apparatus with colored water.
SETUP TIME: 5 min.
 |  |  |  | 
|