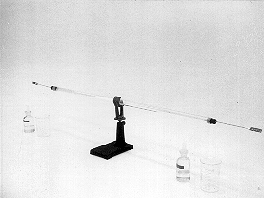

PURPOSE: To show that the diffusion velocity is proportional to the RMS molecular velocity.
DESCRIPTION: Cotton swabs dipped into HCl and NH4OH respectively are inserted into the ends of the tube. HCl gas (molecular weight 36.5) and NH3 or ammonia gas (molecular weight 17) begin to diffuse inward. In less than 15 minutes they meet, forming a ring of ammonium chloride (NH4Cl). The diffusion velocity is proportional inversely to the square root of the molecular mass, because according to equipartition of energy the average molecular speed is inversely proportional to the square root of the molecular mass. This means that the distances traveled by each vapor before they meet are in the proportion:
d(NH3):d(HCl)=SQRT(36.5/17)=3:2 approximately.
SUGGESTIONS:
REFERENCES: (PIRA unavailable.) See Demonstration Reference File for interesting papers on this topic, including application of the diffusion equations to traffic flow.
EQUIPMENT: Glass tube with two stoppers, cotton swabs, HCl and NH4OH.
SETUP TIME: None.
 |  |  |  | 
|