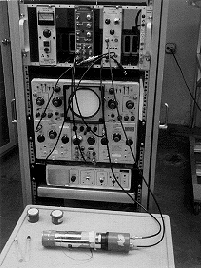

PURPOSE: Show nuclear spectra as evidence of nuclear shells.
DESCRIPTION: Two types of spectra can be seen: alpha, using Am 241 and gamma using Cs 137, Co 60 or Na 22. These spectra are seen using a detector (see below) with a qVt 1024 channel spectrum analyzer with a dual beam oscilloscope to see both the spectrum and the pulses from the detector.
The alpha spectrum is obtained using a small commercial spectroscope which houses the source and a silicon surface barrier detector in a small vacuum chamber.
The gamma spectrum is obtained using a sodium iodide scintillator detector with a phototube base.
Some day we may be able to count electrons, which require a somewhat thicker silicon surface barrier detector, which we do not now have.
SUGGESTIONS: The surface barrier detector can be damaged if operated incorrectly, and the sodium iodide phototube uses a high voltage, so you must get instruction before using this equipment.
REFERENCES: (PIRA 7D10.10)
EQUIPMENT: Roll-around relay rack of NIM bin components as described above.
SETUP TIME: 1 hour.
 |  |  |  | 
|