
Commercial plastic bottles are able to sustain a pressure of 800 KPa but such a pressure will not be necessary. Clean butane condenses at a temperature of -0.5 °C. If the bottle and the gas has a temperature of its environment then the pressure within the bottle may exceed that in a tyre of a vehicle. As a first step let us fill the bottle. The reffiling container should be placed upside down in order to ensure the flow of the gas to the bottle after having opened it with a clamp. The butane entering into the bottle will be of mixture of liquid and gas phase. At the bottom of the bottle there should be liquid butane of 1cm height. For a short time let the butane to boil in order to extrude all the air from the bottle. Then the bottle should be closed. On the bottle we may easily trace the increase of the pressure of butane due to its boiling process. The steaming up of the outher surface is a clear evidence that boiling takes heat from the environment. When the gas attains the temperature of its environment the pressure will be so large that we may hardly be able to compress the bottle. Afterwards the bottle and the gas within should be cooled down below the condensation temperature of the gas. At home we may use the freezer that provides a temperature of -17°C or we may use dry ice or Nitrogen. The volume of liquified butane is smaller than that of the gaseous phase therefore the outer atmospheric pressure squeezes the bottle to a flat shape. Let us place the bottle to the ground and roll over the vehicle onto the bottle. Pay attention to place the bottle right below its center, otherwise side forces would shoot the bottle out of its position. Let us secure the vehicle with hand brake and chocks. Within a short period of time the bottle and the gas within acquires the temperature of its environment and thereupon the pressure increases within the bottle. When this pressure overcomes the pressure in the tyre the vehicle will be lifted.
This experiment can be performed using dry-ice. Under this condition the temperature of the environment can be even below freezing. The bottle should be placed empty under the tyre of the vehicle with its opening left accessible. After the securing the car we may put the dry-ice into the bottle and close it. After heating up the dry-ice converts into a gas of carbon-dioxide, whose pressure may exceed that of the previous experiment. Therefore this experiment should be performed with great care under the supervision of an experienced person.
This experiment can be performed using dry-ice. Under this condition the temperature of the environment can be even below freezing. The bottle should be placed empty under the tyre of the vehicle with its opening left accessible. After the securing the car we may put the dry-ice into the bottle and close it. After heating up the dry-ice converts into a gas of carbon-dioxide, whose pressure may exceed that of the previous experiment. Therefore this experiment should be performed with great care under the supervision of an experienced person.

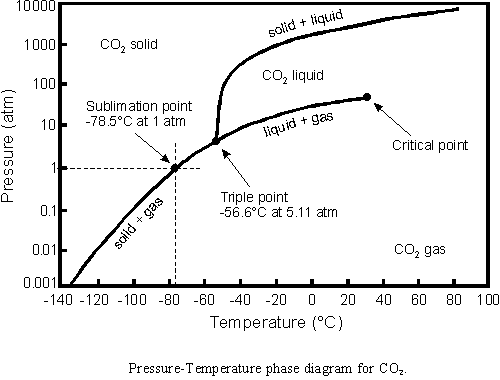
The best is to adapt the bottle cap the following way. A built-in valve enables to control the pressure of the gas in the bottle. The pressure increases with increasing temperature - it can be determined according to the diagram above.
As long as the bottle contains all the three phases - carbon-dioxide is located in its tricritical point - the pressure is well controled (by the Nature). Do not let the solid phase to disappear since else the pressure will increase very fast to an extrame large value. If the solid phase nevertheless disappears you should run into shelter as fast as possible and do not to spend time on unscrewing the cap, since the gas will soon undergo an adiabatic expansion and the pieces of the bottle will fly away in an unpredictable way.
Experiments with the Dry Ice
Carbon-dioxide is an exciting material. We may demonstrate all the phases of matter and the phase transitions as well, without the need for expensive equipment. Carbon dioxide (dry ice) used in the experiment is an extremely exciting material anyway: all physical states and state transformations might be demonstrated with it. This is inspired by the phase diagram of Carbon-dioxide.
Carbon dioxide was first identified in the 1750s by Joseph Black, a Scottish chemist and physician. Carbon dioxide is a colouriess, odourless gas. It occurs in the atmospheres of many planets, including that of the earth. On the earth, all green plants must absorb carbon dioxide from the atmosphere to live and grow. Dry Ice is frozen carbon dioxide, a normal part of our earth's atmosphere. It is the gas that we exhale during breathing and the gas that plants use in photosynthesis. It is also the same gas commonly added to water to make soda water. Dry Ice is particularly useful for freezing, and keeping things frozen because of its very cold temperature: -78.5°C. Dry Ice is widely used because it is simple to freeze and easy to handle using insulated gloves. Dry Ice changes directly from a solid to a gas -sublimation- in normal atmospheric conditions without going through a wet liquid stage. Therefore it gets the name "dry ice."
Sublimation of Dry Ice

Opposite to simple expectations this flask contains dry-ice and not normal ice. Dry-ice is the solid phase of carbon-dioxide. Its temperature is -78.5 degrees Celsius, and its behavior is different from what is normally expected since when warming instead of melting the solid turns slowly to a gas. The resulting gas is colourless and odourless, human sense-organ have no chance to detect it. The presence of the gas can be demonstrated by pulling a baloon over the opening of the flask. Within a short time the balloon will inflate to a sizable extent. Let us cool down the bottom of the falsk. The refrigerant should be liquid nitrogen, so the temperature drops far below -78.5 degrees in the flask. The contraction of the balloon shows the disapearance of the gas. At the bottom of the flask where the temperature is below the -78.5 degrees solid dry-ice appears, whose volume is by orders of magnitude smaller than that of the gas. When all the gas solidifies the flask pulls the balloon in. Let us warm up the flask. This process will induce the appearance of the gas phase and the balloon reappears.
Liquefaction of Carbon Dioxide

Dry ice is placed into an PET plastic cylinder. A valve is closed and pressure in the cylinder increases. When the pressure reaches 511 kPa it stops increasing and liquid CO2 appears. The liquid begins to boil and when all solid CO2 is gone, the pressure increases further. The valve is opened and the pressure drops again, holding constant for a while at 511 kPa. Eventually solid CO2 reforms, the liquid disappears, and the pressure drops completely.
To melt dry-ice seems to be a process out of question since it sublimates. Let us warm up dry-ice within a closed, transparent, pressure resistant vessel. Since the dry-ice is much colder than the room it takes heat from the environment. As the gas resulting from the sublimation is not allowed to leave the vessel the pressure increases. As soon as the pressure reaches the critical value of 511 kPa the wall of the vessel will be moistened by the dry-ice, a little bit later the first drop appears which is a clear evidence of the melting process. The resulting liquid is crystal clear, similar to water. In the course of time as the liquid accumulates it becomes clear that it is boiling. All the three phases of the material can be observed in the vessel: solid, liquid and gaseous together with the process of melting and boiling. The dry-ice can be found in a curious state: it can be concidered to be hot and freezing. The valve at the top of the vessel enables us to let the gas out. Under this condition the liquid starts to boil intensively, so much that it freezes at the same time. The frozen dry-ice due to bubbles released during the boiling process becomes a porosous material. That is called carbonic acid snow that fills up the complete vessel. Close the valve! Then the pressure may increase again and we may observe the collapse of the carbonic acid snow and its melting process.
To melt dry-ice seems to be a process out of question since it sublimates. Let us warm up dry-ice within a closed, transparent, pressure resistant vessel. Since the dry-ice is much colder than the room it takes heat from the environment. As the gas resulting from the sublimation is not allowed to leave the vessel the pressure increases. As soon as the pressure reaches the critical value of 511 kPa the wall of the vessel will be moistened by the dry-ice, a little bit later the first drop appears which is a clear evidence of the melting process. The resulting liquid is crystal clear, similar to water. In the course of time as the liquid accumulates it becomes clear that it is boiling. All the three phases of the material can be observed in the vessel: solid, liquid and gaseous together with the process of melting and boiling. The dry-ice can be found in a curious state: it can be concidered to be hot and freezing. The valve at the top of the vessel enables us to let the gas out. Under this condition the liquid starts to boil intensively, so much that it freezes at the same time. The frozen dry-ice due to bubbles released during the boiling process becomes a porosous material. That is called carbonic acid snow that fills up the complete vessel. Close the valve! Then the pressure may increase again and we may observe the collapse of the carbonic acid snow and its melting process.
Critical opalescense of liquified carbon dioxide

Into this glas tube with thick walls carbon dioxide has been closed. At room temperature two phases may be observed: liquid and gas. This can be made apparent by moving the tube. Heating it up above 31 degrees Celsius, carbon dioxide becomes homogeneously gas. Let it cool down. At a certain moment for a short period all the carbon dioxide becomes opalescent. The reason for this phenomenon is due to strong density fluctuations within the gas that leads to a critical phase. At this point there is no difference between the liquid and the gaseous phase. There is no meniscus, i.e. surface, no surface tension, the latent heat of evaporation is zero and the difference between the density in the liquid and gaseous phase vanishes.